May 2020
UFI – Unique Formula Identifier
What does it mean?
Poison centers play an important role in ensuring the safe use of chemicals and formulating preventive and curative measures in case of poisoning incidents. They provide medical advice to general consumers and physicians on health emergencies, arising from exposure to hazardous chemicals or to other toxic agents.
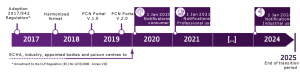
CLP Article 45(4): as per 20 January 2012 the Commission shall carry out a review to assess the possibility of harmonizing the information referred to in paragraph 1, including establishing a format for the submission of information by importers and downstream users to appointed bodies.
Furthermore, in the commission Regulation (EU) 2017/542 on harmonized information relating to emergency health response/CLP Annex VIII is stated:
- Obligation for importers and downstream users placing hazardous mixtures on the market to notify national appointed bodies
- Phased deadlines by 1 January
- 2020 for consumer use
- 2021 for professional use
- 2024 for industrial use
- 2025 for products having already been submitted before the three deadlines above (marks end of transition period)
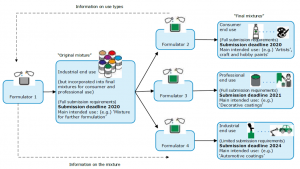
Practically this means, that all mixtures classified as hazardous for human health or physical hazard have to be equipped with a UFI. The UFI is a unique code, which is integrated in the SDS and has to be printed on the label of the product. It will create an unambiguous link between a mixture placed on the market and the information on that specific mixture submitted to poison centres, so that the chemical formulation of the product can be precisely and rapidly identified. A precise identification is necessary to provide appropriate curative measures in the case of an emergency call. This requirement applies throughout the European Economic Area, which comprises 28 Member States of the EU and Norway, Iceland and Liechtenstein.
We have to evaluate which Würth products will fall under the new CLP Annex VIII and also if they are classified as consumer or professional use within the respective country. This is very important since the deadline for consumer use items is set an earlier date than the one for professional use. Items, which have already been registered on a local level in the past, have to be equipped with a UFI as soon as there is a relevant change in classification of the product, but by 1 January 2025 at the latest. All new items have to be equipped with a UFI in accordance as per 2020 or 2021, depending on its defined use.
Additionally Würth (Product Management) has to assign to each of these products the corresponding packaging type and the uses.
As soon as 3E has gotten a UFI code from ECHA, the code will then be transmitted to Würth. Würth will then make sure that the code is placed on the labels and that these are submitted to the supplier. It is our goal not to have to scrap any labels while introducing the UFI and we will do our utmost to coordinate the project accordingly.